Fig. 1. Yeast two-hybrid mating strategy. A plasmid expressing a library prey protein (Y) fused to the GAL4 transcrition activation domain (AD) is transformed into a yeast cell of mating type α. Upon combination (mating) with a yeast cell of the opposite mating type (A) expressing the bait protein (X) fused to the GAL4 DNA binding domain (BD), a diploid yeast cell is formed, allowing the protein-protein interaction and consequently transcription of the reporter genes to take place. This is assessed by detecting growth on media lacking histidine and adenine, the development of a blue/green color by a plate assay detecting α-galactosidase, and detection of β-galactosidase by a LacZ filter-lift assay.
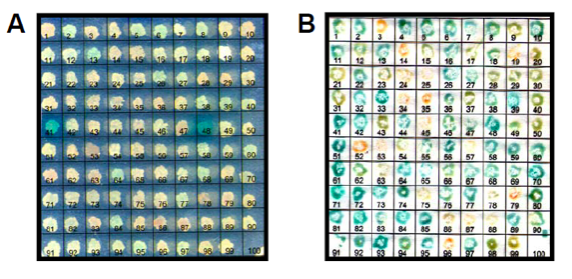
Fig. 2. Assessment of the results from yeast two-hybrid screening. (A) Detection of activation of the MEL1 reporter gene by a colorimetric X-α-gal plate assay, detecting α-galactosidase production and secretion by the growing yeast colonies. (B) Detection of activation of the LacZ reporter gene by a colorimetric X-β-gal plate assay, detecting β-galactosidase production in the yeast cytoplasm by a filter-lift assay.
Yeast Two-Hybrid
Identification of binary protein-protein interactions is a crucial step in determining the molecular context and functional pathways of proteins. The yeast two-hybrid system a powerful method to reveal information about transient interactions, about the binary protein pairs or about the interacting epitopes. We have employed an optimized GAL4-based yeast two-hybrid system (Fields, S., Song, O., Nature, 1989) to dissect the cilium-associated protein complexes. In this system a "bait" protein is expressed as a fusion to the GAL4 DNA binding domain, and this is used to screen a "prey" library of cDNA expressing the encoded proteins fused to the GAL4 activation domain, by coexpression in yeast (Fig. 1). Both bait and prey proteins are translocated to the yeast nucleus due to the presence of a nuclear localization signal. When a bait protein interacts with a prey protein, the DNA binding domain that is attached to the promoter region of a reporter gene is brought in close vicinity to the transcription activation domain, thus restoring functional transcription factor activity and activating the reporter gene. The reporter gene activation for HIS3 and ADE1 is detected by growth on selective plates and the reporter gene activation of MEL1 and LacZ is detected by a colorimetric galactosidase assays (Fig. 2).